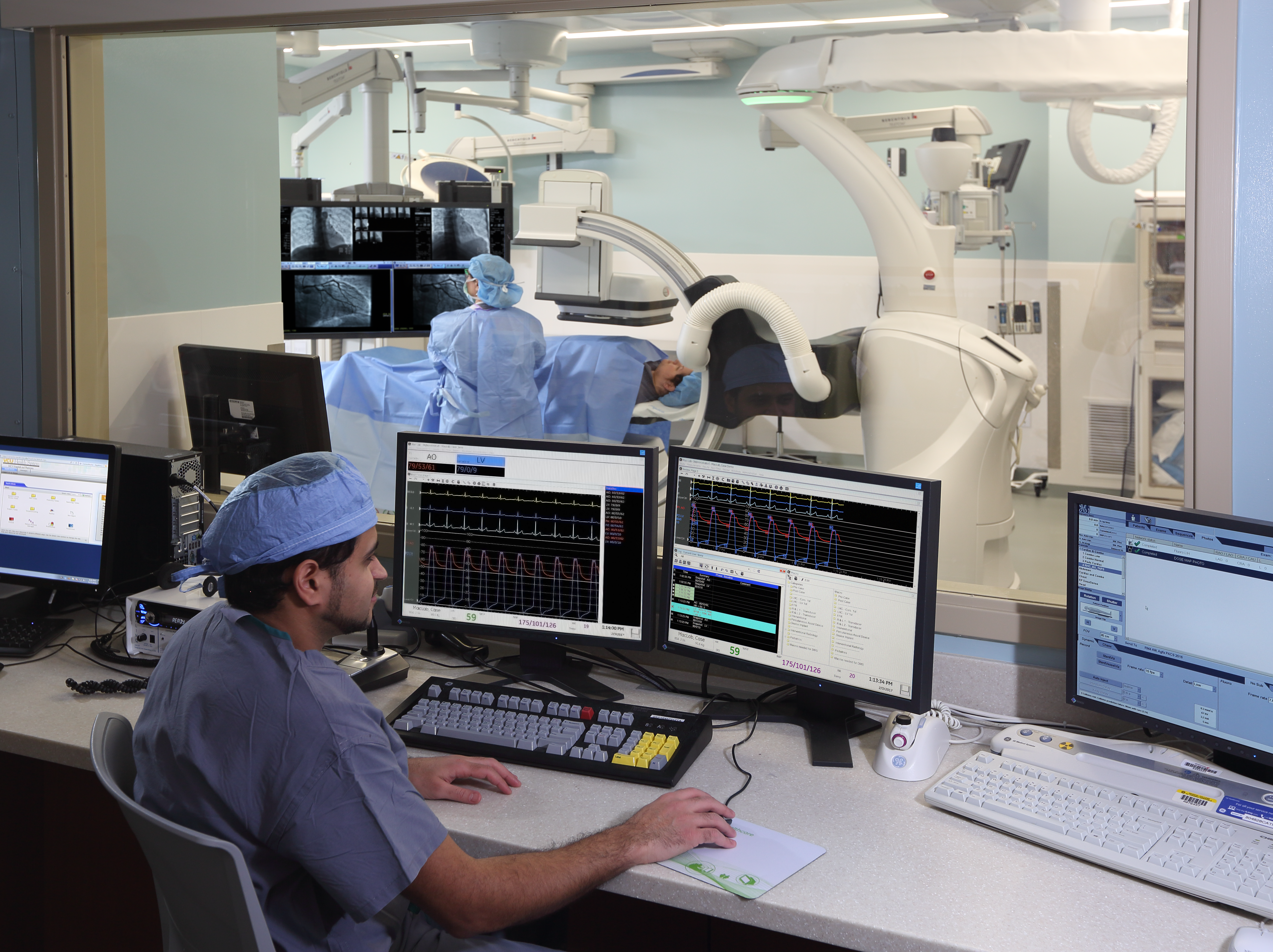
For patients with symptomatic and severe aortic stenosis (AS), transcatheter implantation or replacement of the aortic valve (otherwise known as TAVI/ TAVR) is being used more and more as an alternative to open surgery. Many testing modalities—including ultrasound, MRI, and echocardiography—support these interventions. The ECG, which has long proved useful in many areas of medicine, also has a key role to play in evaluating patients for these procedures, guiding management choices, and providing prognostic information before patients are taken to the cath lab for valve replacement.
The latest U.S. valvular heart disease guidelines place the ECG early on in the initial process of evaluating patients with known or suspected valve disease. These guidelines state that a patient's medical history and physical exam findings should be considered alongside the results of noninvasive testing such as X-rays, transthoracic echocardiography, and the ECG—which evaluates rhythm and LV function and looks for hypertrophy.1
The ECG as a first look
Though use of other modalities has grown, the ECG remains an essential tool in the evaluation of a patient for AS, as reflected by its inclusion in the guidelines, and this is underscored in recent research.
A review in the Journal of Electrocardiography, for instance, focused on the importance of LV strain on the ECG—indicated by "down-sloping, convex ST-segment depression with asymmetric T-wave inversion in leads V5 and V6"—as a reflection of fibrosis resulting from subendocardial ischemia in patients with AS.2 Because this finding has been associated with a greater risk of adverse cardiovascular events irrespective of the presence of LV hypertrophy, it should warrant a response from the treating physician. This might include more-aggressive control of hypertension or even consideration of an intervention like TAVI in borderline cases, the authors say.
There's also emerging research showing how artificial intelligence-enabled ECG (AI-ECG) can be used to identify patients with a variety of cardiovascular diseases—including AS—earlier, potentially leading the way to improved clinical outcomes down the line.
A group at the Mayo Clinic has performed much of this work, and one of their recent studies, published in the European Heart Journal – Digital Health, demonstrated that an AI-ECG model identified previously undiagnosed moderate-severe AS in more than one-quarter of a large cohort.3 Moreover, the information gleaned from the ECG in this manner correlated with key echocardiographic parameters reflective of AS severity, diastolic dysfunction, and LV hypertrophy, including aortic valve area, peak velocity, mean pressure gradient, LV mass index, and left atrial volume index (but not LV ejection fraction or stroke volume index).
The promise of AI-ECG was affirmed by another study showing that an ECG-based machine learning model performed well for identifying a composite of structural heart diseases that included AS and others in patients with previously unrecognized issues.4
Editors from the Journal of the American College of Cardiology point out that some questions remain unanswered. Can the AI-ECG model be generalized across different centers, ECG machines, data management systems, and populations? What level of customization is needed in different settings? How will AI-ECGs perform over time? "These questions clearly highlight the need for multisite validation studies, large prospective studies, implementation studies (integrating such models in routine clinical workflow), and randomized clinical trials evaluating the impact of AI-based screening on clinical outcomes among patients with valvular heart disease," editors write.5
How the ECG impacts other tools for TAVI evaluation
An expert consensus decision pathway from the American College of Cardiology (ACC) recommends a multidetector computed tomography (CT) for patients being evaluated for TAVI, and ECG has a role to play here as well.6 Although scanning protocols vary, a multidetector CT typically consists of two main components—ECG-gated acquisition of the aortic annulus and aortic root and then a scan of the full chest, abdomen, and pelvic area, which doesn't usually require ECG gating.
Once a provider decides to move ahead with TAVI/ TAVR for a specific patient, the preprocedural evaluation generally begins with transthoracic echocardiography, but if additional information is needed, cardiac MRI and ECG-gated multidetector CT can provide insights into valve anatomy and function, according to the ACC.
Guidance from the Society of Cardiac Computed Tomography (SCCT) also highlights how the ECG can support the evaluation of patients prior to TAVI/ TAVR.7 Acquisition strategies and scanning protocols differ depending on scanner manufacturers, systems, and hospital preferences, the SCCT says, but "the key component of all approaches is an ECG-synchronized computed tomographic angiography (CTA) data set that covers at least the aortic root in order to provide artifact free anatomical information of the aortic root," followed by a non-ECG-gated scan of the access vasculature.
As Pablo Abbona, MD, a physician from the University of California, Irvine, notes, ECG-gated CT can help ensure that a patient is a good candidate for TAVI and guide the selection of the prosthesis and treatment approach, which may lessen the risk of procedural complications like prosthesis embolization and paravalvular regurgitation.8
ECG-gating can also enhance MRI evaluation prior to TAVI, as discussed in an Insights Into Imaging review.9
Aortic valve intervention: Choosing between TAVI and SAVR
As transcatheter and surgical replacement of the aortic valve (SAVR) both have Class I indications for treating patients with symptomatic and severe aortic stenosis, the choice between the two options comes down to deliberations by multidisciplinary heart teams; these teams typically consider various clinical and anatomic factors, as well as patient preference, according to an article in the European Heart Journal (EHJ).10
The authors note that ECG may aid in assessing the multitude of factors that come into play when deciding whether to use TAVI or SAVR. Coronary heart disease ECG findings may complement other tools like invasive angiography and coronary CT angiography to determine the complexity of coronary artery disease, with more complex diseases possibly pushing the decision more toward surgery.
Risk of new conduction disturbances such as complete heart block after TAVI requiring pacemaker implantation is another aspect to consider when weighing transcatheter against surgical replacement of the valve—and an ECG or preprocedural CT can help here as well. This risk varies depending on anatomic features, device type, and TAVR technique.
"It remains uncertain whether the choice of SAVR may further reduce the risk of new conduction disturbances leading to permanent pacemaker implantation compared with TAVI, especially with high implantation techniques," the EHJ authors state. "Notwithstanding, the risk of conduction disturbances is an important consideration for treatment selection in patients at high risk for conduction disturbances, and SAVR emerges as the preferred option particularly in young patients with long life expectancy."
Preprocedural ECG as an indicator of pacemaker and prognosis after TAVI
Baseline ECG findings will provide physicians with a more robust picture of the risk of conduction disturbances that may require permanent pacemaker implantation—as well as the likelihood of adverse clinical outcomes—after TAVI.
In a paper in Circulation, the authors note that pre-existing right bundle branch block (RBBB) "is probably the strongest, most consistent clinical predictor of PPM [permanent pacemaker implantation]," since more than half the studies examining the issue have found RBBB to be predictive.11
In one such study published in the Journal of the American College of Cardiology, the presence of RBBB, first-degree atrioventricular (AV) block, or left anterior hemiblock before TAVI was associated with a greater need for permanent pacemaker implantation after the procedure.12
Other studies have identified baseline ECG predictors of not only permanent pacemaker implantation, but also adverse clinical outcomes after the procedure. The OCEAN-TAVI registry investigators, for instance, have identified pre-existing RBBB as an independent predictor of cardiovascular mortality after multivariate adjustment (HR 2.65) in one study, and the absence of ECG-detected LV hypertrophy as a predictor of all-cause mortality after TAVI.13,14
And in another study, RBBB detected at baseline correlated with greater risks of both all-cause death (HR 1.31) and CV death (HR 1.45) after TAVI.15
Pre-existing left bundle branch block (LBBB) was associated with a greater risk of permanent pacemaker implantation, but not of mortality or heart failure hospitalization in one study,16 whereas another analysis demonstrated that the presence of advanced interatrial block was a predictor of all-cause mortality (HR 1.48) and a composite of death, stroke, or new Afib.17 As the authors of the latter study note, "A simple inexpensive measurement of surface ECG could contribute to better risk stratification in patients who are treated with transcatheter aortic valve implantation."
Findings from clinical research, anatomic factors, and patient preference can lead to improved decision-making for patients being considered for TAVI.
Resources:
1. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. December 2020;143(5):e72-e227. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000923
2. Saeed S, Wasim D, Mohamed Ali A, et al. The electrocardiogram: still a useful marker for LV fibrosis in aortic stenosis. Journal of Electrocardiology. March-April 2021;65:82-87. https://www.sciencedirect.com/science/article/abs/pii/S0022073621000169
3. Ito S, Cohen-Shelly M, Attia ZI, et al. Correlation between artificial intelligence-enabled electrocardiogram and echocardiographic features in aortic stenosis. European Heart Journal – Digital Health. May 2023;4(3):196-206. https://academic.oup.com/ehjdh/article/4/3/196/7031475
4. Ulloa-Cerna AE, Jing L, Pfeifer JM, et al. rECHOmmend: an ECG-based machine learning approach for identifying patients at increased risk of undiagnosed structural heart disease detectable by echocardiography. Circulation. July 2022;146(1):36-47. https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.121.057869
5. Pandey A, Adedinsewo D. The future of AI-enhanced ECG interpretation for valvular heart disease screening. Journal of the American College of Cardiology. August 2022;80(6):627-630. https://www.jacc.org/doi/full/10.1016/j.jacc.2022.05.034
6. Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology task force on clinical expert consensus documents. Journal of the American College of Cardiology. March 2017;69(10):1313-1346. https://www.jacc.org/doi/10.1016/j.jacc.2016.12.006
7. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. Journal of Cardiovascular Computed Tomography. January 2019;13(1):1-20. https://www.journalofcardiovascularct.com/article/S1934-5925(18)30536-7/fulltext
8. Abbona P. ECG gated CT of transcatheter aortic valve replacement (TAVR) planning. Radiology.UCI.edu. https://radiology.uci.edu/2020/12/30/ecg-gated-ct-of-transcatheter-aortic-valve-replacement-tavr-planning/. Accessed April 25, 2023.
9. Chaturvedi A, Hobbs SK, Ling FS, et al. MRI evaluation prior to transcatheter aortic valve implantation (TAVI): when to acquire and how to interpret. Insights Into Imaging. February 2016;7:245-254. https://link.springer.com/article/10.1007/s13244-016-0470-0
10. Windecker S, Okuno T, Unbehaun A, et al. Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? European Heart Journal. April 2022;43(29):2729-2750. https://academic.oup.com/eurheartj/article/43/29/2729/6572863
11. Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. September 2017;136(11):1049-1069. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.117.028352
12. Siontis GCM, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. Journal of the American College of Cardiology. July 2014;64(2):129-140. https://www.sciencedirect.com/science/article/pii/S073510971402436X
13. Watanabe Y, Kozuma K, Hioki H, et al. Pre-existing right bundle branch block increases risk for death after transcatheter aortic valve replacement with a balloon-expandable valve. JACC: Cardiovascular Interventions. November 2016;9(21):2210-2216. https://www.sciencedirect.com/science/article/pii/S1936879816314248
14. Koga M, Izumo M, Yoneyama K, et al. Prognostic value of electrocardiographic left ventricular hypertrophy after transcatheter aortic valve implantation: insights from the OCEAN-TAVI registry. October 2023;204:130-139. https://europepmc.org/article/med/37541149
15. Auffret V, Webb JG, Eltchaninoff H, et al. Clinical impact of baseline right bundle branch block in patients undergoing transcatheter aortic valve replacement. JACC: Cardiovascular Interventions. August 2017;10(15):1564-1574. https://www.sciencedirect.com/science/article/pii/S1936879817309937
16. Fischer Q, Himbert D, Webb JG, et al. Impact of preexisting left bundle branch block in transcatheter aortic valve replacement recipients. Circulation: Cardiovascular Interventions. November 2018;11:e006927. https://www.ahajournals.org/doi/10.1161/CIRCINTERVENTIONS.118.006927
17. Vicent L, Fernández-Cordón C, Nombela-Franco L, et al. Baseline ECG and prognosis after transcatheter aortic valve implantation: the role of interatrial block. Journal of the American Heart Association. November 2020;9(22):e017624. https://www.ahajournals.org/doi/10.1161/JAHA.120.017624